Overview
Porphyrins are generally planar and rigid molecules. We aim at synthesizing porphyrinoid molecules that possess much altered electronic properties than what is found in regular porphyrins and chlorins. This may be accomplished through a step-wise modifications to a non-pyrrolic moiety (for their syntheses see here; their optical properties see here). Two factors can be attributed to this electronic modulation: a direct electronic influence of the new ring and through the modulation of the conformation of the PMP. The general electronic influence of the conformation of the porphyrinoids on their electronic properties is well-recognized, though many details remain to be discovered.
For the codification of the structure-relationships of PMPs that will eventually help to design porphyrinic chromophores with designed photophysical properties, we study – often in collaboration with specialty groups – the conformation and conformational flexibility of PMPs using, for instance, multinuclear, variable temperature, 1D and 2D NMR techniques, electrochemistry, CD spectroscopy, computations, and single crystal X-ray diffractometry.
PMP Conformation and Conformational Flexibility
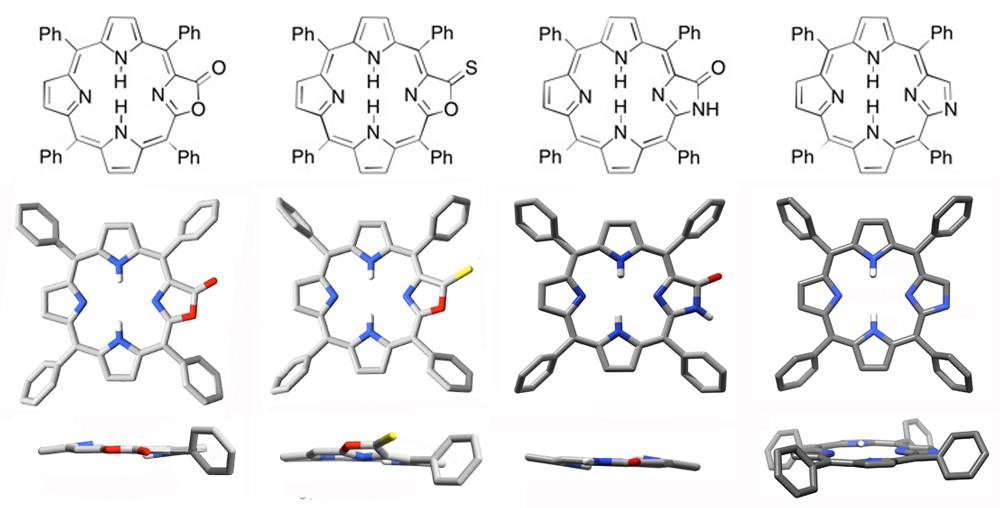
The replacement of a pyrrole by an unsaturated non-pyrrolic five-membered ring has little to no affects on the planar conformation of the parent porphyrin (Org. Biomol. Chem. 2013, 11, 4613-4621. Org. Biomol. Chem. 2013, 11, 3616–3628. J. Org. Chem. 2012, 77, 6480–6494. Org. Biomol. Chem. 2011, 9, 2306–2313.).
Even the reduction and/or functionalization of the five-membered ring may have a minimal influence on the conformation of the PMP, as a number of examples of oxazolochlorins demonstrate ( J. Org. Chem. 2012, 77, 6480–6494. J. Org. Chem. 2012, 77, 6199–6207.).
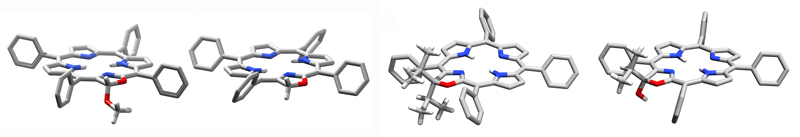
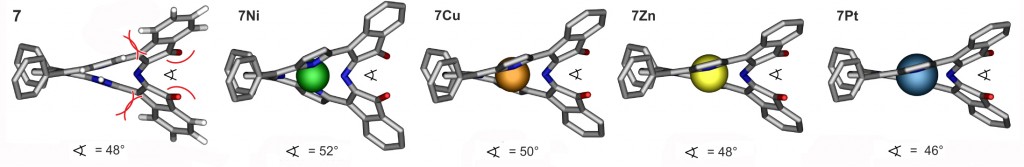
In fact, the replacement of two pyrroles by oxaxoles has only a minimal influence on the conformation of the resulting bacteriochlorin analogues (J. Org. Chem. 2013, 78, 2840–2852.). On the other hand, the replacement of a pyrrole by a six-membered morpholine ring results in a drastic distortion from planarity that, in the free base chromophores, is modulated by the number and type of substituents on the morpholine ring (J. Am. Chem. Soc. 2011, 133, 8740–8752.) and the presence of a central metal present.
Many of the chiral, ruffled conformers are conformationally stable and thus their enantiomers can be searated by chiral HPLC (J. Am. Chem. Soc. 2011, 133, 8740–8752.).
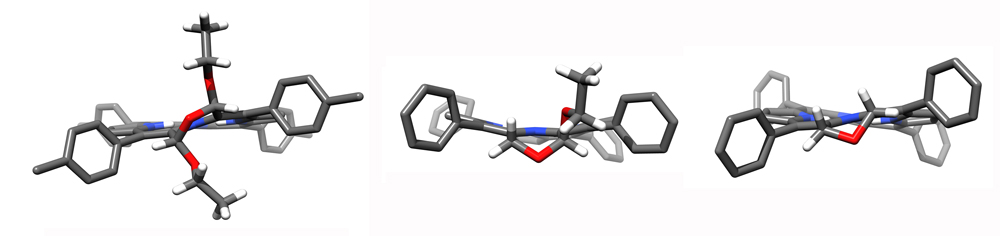
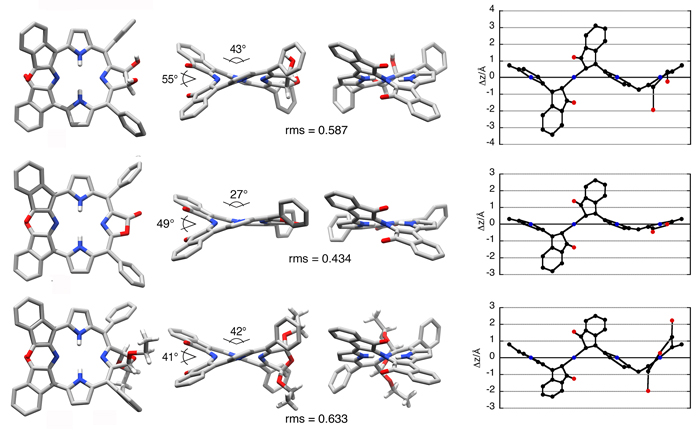
The introduction of an additional ß,ß’-reduction or a second morpholine ring leads to the expression of severely ruffled conformations in these bacteriochlorin analogues (Angew. Chem. 2012, 124, 5856–5859.).
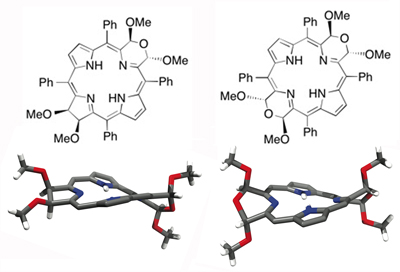
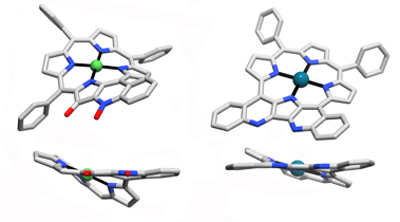
Indaphyrins possess a cleaved ß,ß’-bond and the former ß-carbons are fused to the flanking meso-phenyl groups forming indanone moieties (Org. Biomol. Chem. 2004, 2, 1484–1491.). Their conformations are also ruffled, central metal dependent, conformationally surprisingly stable, and their helimeric enantiomers can be chirally resolved (Eur. J. Org. Chem. 2015, 3913–3922. Inorg. Chem. 2009, 48, 4067–4074.).
When further modified, the conformation of the parent indaphyrin is further modulated, in sometimes surprising ways, suggestive of a high conformational flexibility of these indaphyrin derivatives (Chem.–Eur. J. 2015, accepted for publication.), yet they are not as flexible as to allow racemization of their chiral conformations (Eur. J. Org. Chem. 2015, 3913–3922.).
Conformation and Conformational Flexibility of Other Porphyrinoids
Quinoline-annulated porphyrins possess a porphyrin-type chromophore but, on account of their extended π-systems, much altered electronic properties compared to regular porphyrins. In addition, their conformation is being modulated by the annulation. We have studied the conformation of the quinine-annulated porphyrins in detail (J. Org. Chem. 2015, 80, 499-511.).
Because of a steric interaction between the o-hydrogens on the meso-phenyl groups with the neighboring ß-hydrogen, meso-phenyl groups are forced to adopt an essentially orthogonal conformation with respect to the plane of the porphyrin. Therefore they are not in conjugation with the porphyrinic chromophore (we have studied this effect also in corroles: Photochem. Photobiol. 2014, 90, 402–414. ). The smaller and thus streakily less demanding thienyl groups can adopt a much more co-planar conformation with the porphyrnic chromophore. As a consequence, they have a much larger influence on the electronic properties of the porphyrinic chromophore, especially if the chromophore is a bit distorted through, for instance, protonation.
We have also utilized thienylporphyrins, and their metal complexes, in chemosensing (Biosensors Bioelectronics 2010, 26, 504–510. Sens. Actuators, B 2010, 147, 191–197.) and electrocatalytic applications (J. Power Sources, 2014, 257, 246–253. J. Phys. Chem. C 2010, 114, 8633–8638.).