A chemosensor (molecular sensor) is a molecule that interacts with a specific analyte to produce a detectable change. Molecular sensors exhibit molecular recognition properties or undergo analyte-specific reactions that are then reflected in a signal. We generally detect the change of the optical (UV-vis or fluorescence spectra) or electrochemical properties of functionalized porphyrinoids or coumarins to detect the presence of an analyte.
Full list of publications.
.
Explosives Sensing
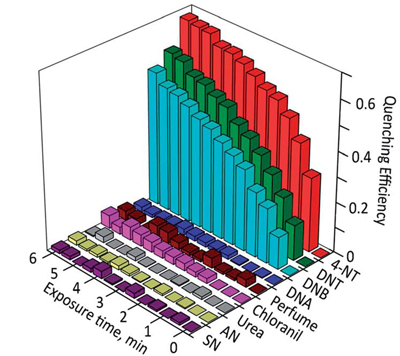 In collaboration with Prof. Yu Lei (UConn, Department of Chemical and Bimolecular Engineering) we developed a wide range of explosives chemosensing methods (J. Mater. Chem. A 2014, 2, 14613-14621. Chem. Commun. 2012, 48, 9903–9905. Sens. Actuators, B 2010, 147, 191–197.). The increased risk of terrorist bombing, IDEs, the wide-spread use of mines, and water and soil contamination by explosives require the development of rapid, simple, and sensitive methods to detect the common explosives.
In collaboration with Prof. Yu Lei (UConn, Department of Chemical and Bimolecular Engineering) we developed a wide range of explosives chemosensing methods (J. Mater. Chem. A 2014, 2, 14613-14621. Chem. Commun. 2012, 48, 9903–9905. Sens. Actuators, B 2010, 147, 191–197.). The increased risk of terrorist bombing, IDEs, the wide-spread use of mines, and water and soil contamination by explosives require the development of rapid, simple, and sensitive methods to detect the common explosives.
.
Oxygen Sensing
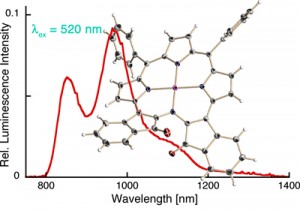 Optical oxygen sensing by Pt(II) or Pd(II) porphyrinoids relies on the oxygen partial pressure-dependent dynamic (Stern-Vollmer) quenching of the phosphorescence of the sensor dye. Many of our porphyrinoids prepared possess much altered electronic properties compared to regular porphyrins or chlorins. For instance, the indaphyrin Pt(II) complexes possess extremely red-shifted emission properties. This suggest that they might also possess altered Stern-Volmer quenching properties, perhaps leading to particularly sensitive oxygen sensing systems of utility as oxygen sensitive paints used in the imaging of air flow. For instance, the indaphyrin Pt(II) complexes possess extremely red-shifted emission properties (Inorg. Chem. 2009, 48, 4067–4074.). Also, luminescent oxygen sensors for biological media are sought-after, and our long-wavelenght absorbing dyes may have an advantage over the traditionally used dyes.
Optical oxygen sensing by Pt(II) or Pd(II) porphyrinoids relies on the oxygen partial pressure-dependent dynamic (Stern-Vollmer) quenching of the phosphorescence of the sensor dye. Many of our porphyrinoids prepared possess much altered electronic properties compared to regular porphyrins or chlorins. For instance, the indaphyrin Pt(II) complexes possess extremely red-shifted emission properties. This suggest that they might also possess altered Stern-Volmer quenching properties, perhaps leading to particularly sensitive oxygen sensing systems of utility as oxygen sensitive paints used in the imaging of air flow. For instance, the indaphyrin Pt(II) complexes possess extremely red-shifted emission properties (Inorg. Chem. 2009, 48, 4067–4074.). Also, luminescent oxygen sensors for biological media are sought-after, and our long-wavelenght absorbing dyes may have an advantage over the traditionally used dyes.
.
High pH Sensing
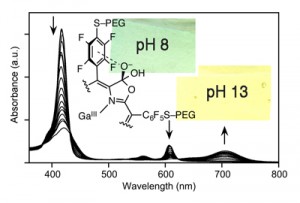 Porpholactones were discovered and developed by us as high pH (between pH ~10 and 13.5) and alkoxide optical sensors (Analyst 2010, 135, 4505–4508.). The influence of the aryl groups, the central metal, and ß‑nitro groups on the sensing profiles was evaluated (J. Porphyrins Phthalocyanines 2013, 17, 836–849.). Water-soluble derivatives were prepared and also incorporated into Nation membranes, thus generating stable optode membranes for the detection or monitoring of extremely high OH– concentrations (Analyst 2015, 140, 190-196.).
Porpholactones were discovered and developed by us as high pH (between pH ~10 and 13.5) and alkoxide optical sensors (Analyst 2010, 135, 4505–4508.). The influence of the aryl groups, the central metal, and ß‑nitro groups on the sensing profiles was evaluated (J. Porphyrins Phthalocyanines 2013, 17, 836–849.). Water-soluble derivatives were prepared and also incorporated into Nation membranes, thus generating stable optode membranes for the detection or monitoring of extremely high OH– concentrations (Analyst 2015, 140, 190-196.).
Together with Prof. Masoud Ghandehari (NYU-Poly, Department of Civil Engineering) and Gamal Khalil (U of Washington), we applied our pH sensing dyes to the pH mapping of concrete by monitoring the pH-dependent reactions, collectively termed ‘alkaline silica reactions’, that contribute to the premature aging of concrete structures.
.
Other Porphyrinoid-Based Chemosensors and Chemodosimeters
 Certain porpholactone derivatives also show a high sensitivity toward the optical detection of cyanide (CN–) in aqueous solutions. Stable and reversible optode membranes were realized (Org. Biomol. Chem. 2014, 12, 3991–4001.).
Certain porpholactone derivatives also show a high sensitivity toward the optical detection of cyanide (CN–) in aqueous solutions. Stable and reversible optode membranes were realized (Org. Biomol. Chem. 2014, 12, 3991–4001.).
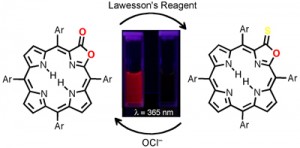
Thionolactones are non-fluorescent. They can be (irreversibly) converted specifically with hypochloride (OCl–) to the corresponding strongly fluorescing porpholactone derivatives. This is the basis for the evaluation of thionolactones as chemidosimeters for hypochlorite, work that was published in collaboration with the group of Prof. Jun-Long Zhang (College of Chemistry and Molecular Engineering, University of Peking) (Org. Biomol. Chem. 2013, 11, 4613-4621.).
.
Zinc Sensing
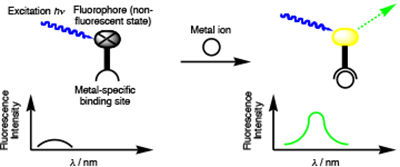 A metal-ion chemosensor comprises a metal recognition and a signal-transduction domain that is triggered upon ion binding. For instance, metal chelation may result in fluorescence enhancement. Zinc is used by hundreds of proteins to stabilize structure or to serve catalytic functions. Zinc deficiency results in a wide range of pathologies, presumably due to the loss of zinc from these proteins. However, the linkages between the basic biochemistry of zinc and the physiology of its deficiency are not well established. Zinc homeostasis, the combined mechanisms controlling the concentration of zinc, even on the cellular level is
A metal-ion chemosensor comprises a metal recognition and a signal-transduction domain that is triggered upon ion binding. For instance, metal chelation may result in fluorescence enhancement. Zinc is used by hundreds of proteins to stabilize structure or to serve catalytic functions. Zinc deficiency results in a wide range of pathologies, presumably due to the loss of zinc from these proteins. However, the linkages between the basic biochemistry of zinc and the physiology of its deficiency are not well established. Zinc homeostasis, the combined mechanisms controlling the concentration of zinc, even on the cellular level is 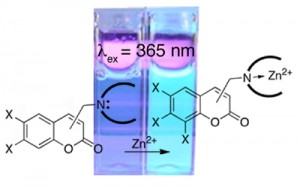 not well understood. In part, the paucity of knowledge can be attributed to the spectroscopically silent nature of this closed shell d10-metal ion, complicating its direct observation (Lim, N.C.; Freake, H. C.; Brückner, C. ‘Illuminating Zinc in Biological Systems’ Chem.–Eur. J. 2005, 11, 38–49.).
not well understood. In part, the paucity of knowledge can be attributed to the spectroscopically silent nature of this closed shell d10-metal ion, complicating its direct observation (Lim, N.C.; Freake, H. C.; Brückner, C. ‘Illuminating Zinc in Biological Systems’ Chem.–Eur. J. 2005, 11, 38–49.).
We contributed to the field by synthesis and evaluation of a family of coumarin-based sensors (Inorg. Chem. 2005, 44, 2018–2030. Chem. Commun. 2004, 1094–1095. Bioorg. Med. Chem. Lett.2003, 13/14, 2251–2254. ).
We are not currently pursuing this line of research.
.
Iron Sensing
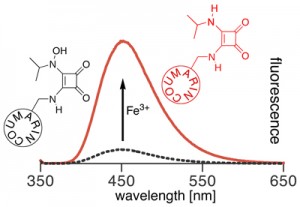 Iron is the fourth most abundant element in the Earth’s crust, but occurs at extremely low concentrations in the ocean due to the low solubility of the iron(III) hydroxides. As a result of its low solubility, iron is the limiting nutrient for primary production in many high-nutrient areas in the ocean. Determinations of the spatial variability of bioavailable iron concentrations are therefore essential to understand mechanisms driving primary production. However, the extremely low concentration of iron and its paramagnetism quenching the fluorescence of many fluorophores complicate its analysis.
Iron is the fourth most abundant element in the Earth’s crust, but occurs at extremely low concentrations in the ocean due to the low solubility of the iron(III) hydroxides. As a result of its low solubility, iron is the limiting nutrient for primary production in many high-nutrient areas in the ocean. Determinations of the spatial variability of bioavailable iron concentrations are therefore essential to understand mechanisms driving primary production. However, the extremely low concentration of iron and its paramagnetism quenching the fluorescence of many fluorophores complicate its analysis.
At the time, iron(III)-specific chemosensors were generally based on the fluorescence quenching effect of the paramagnetic iron(III) ion, i.e. the measurements are based on the disappearance of a signal. We aimed on developing CHEF-type switch-on chemosensors specific for the direct fluorometric detection of iron(III). Instead, we achieved the synthesis of one of the first fluorescence switch-on chemodosimeters for Fe(III) (Inorg. Chem. 2009, 48, 1173–1182.). We also gained insight into the chemistry of the intriguing squaric acid amides (J. Org. Chem. 2003, 68, 9233–9241.).
We are not currently pursuing this line of research.