Utility of Long-Wavelength Absorbing and Emitting Porphyrinic Chromophores
Depending on the particular PMP and associated photophysical properties, we are targeting a number of applications:
Photodynamic Therapy
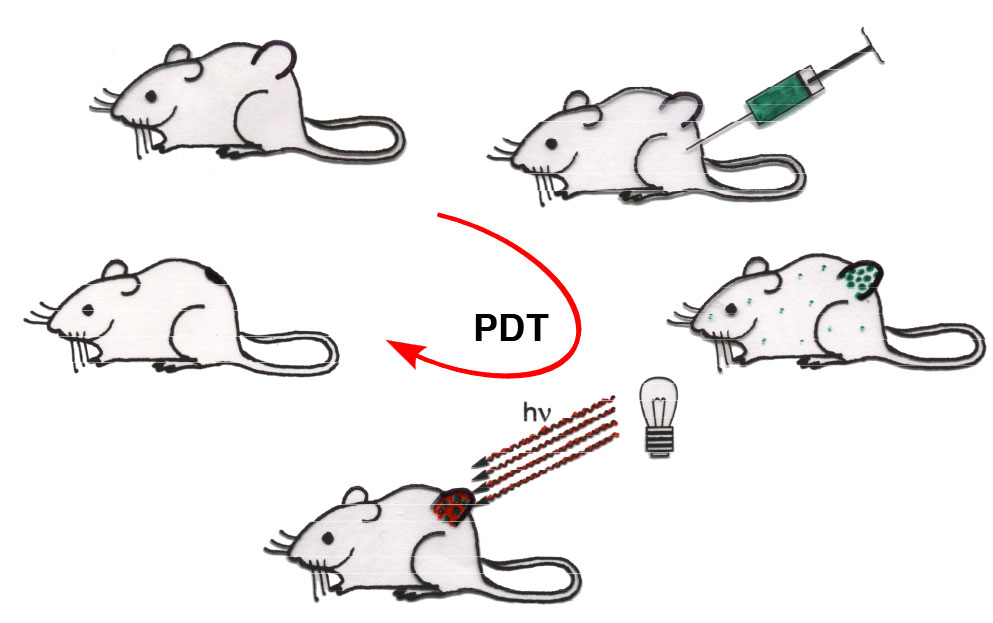
Porphyrinoids are the most promising class of chromophores to be tested as drugs in PDT, a treatment modality that uses the combination of a non-toxic drug, oxygen, and light to kill unwanted tissue. Light is absorbed by a porphyrinoid whereupon it gets excited. One relaxed into a triplet state, it may undergo an energy- and spin-exchange with molecular triplet oxygen (3O2), recovering the ground state photosensitizer and generating the highly reactive and thus cytotoxic species singlet oxygen (1O2).
Systemic treatment of a cancer patient with the photosensitized allows it to accumulate in the metabolically most active cancerous tissue. Only when light shown onto the tumor it generates the cytotoxic singlet oxygen, causing oxidative stress and apoptosis, and the tumor is resorbed. Non-irradiated areas are not affected.
PDT drugs for the treatment of solid tumors should absorb light within the ‘photo-therapeutic window’ of tissue (~700-900 nm, i.e., red and near-IR light), the wavelength range for which tissue is most transparent, therefore allowing deep treatment depth. PDT has been used as a cancer treatment modality, immunomodulation method, as well as photo-antimicrobial or photo-antifungal treatment.
We have identified the class of oxochlorins has chromophores with efficacy in PDT in cell and mouse models.
Fluorescence Imaging
Porphyrinic molecules are frequently strongly fluorescent, owing to their use in the in vivo imaging of tumors, 3D tissue imaging at sub-µm resolution, and fluorescence tagging of biomolecules. In general, all imaging and (chemo)sensing applications in biological and opaque media also benefit from NIR light absorption.
Photoacoustic Imaging
Photoacoustic imaging (PAI) is a non-invasive imaging modality that combines the advantages of high optical contrast and ultrasound (sub-mm) spatial resolution. Absorption of a light pulse by a chromophore causes a rapid and transient mK rise in temperature, leading to a localized thermoelastic expansion. As a pulsed ns laser beam is scanned through an object, the emitted ultrasonic wave profile is acquired. With hemoglobin as an endogenous chromophore, PAI scans of tissue are used to reconstruct 2D/3D vascular maps. To acquire PAI images of deeply-seated lesions, the use of exogenous contrast agents absorbing in the spectroscopic window of tissue (~700 to 900 nm) is needed.
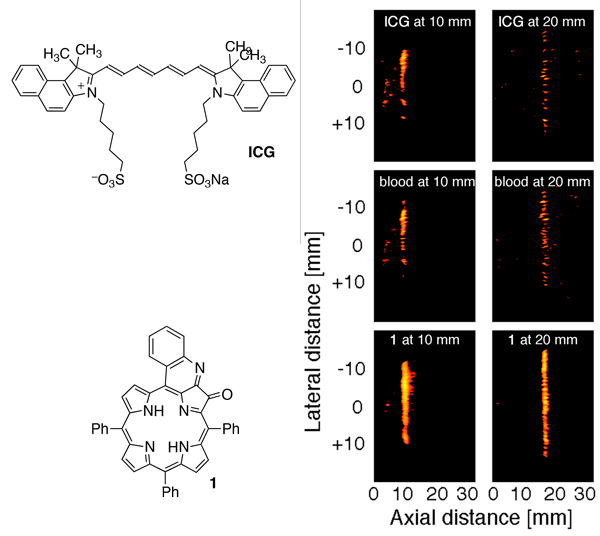
In collaboration with Prof. Quing Zhu (UConn, Department of Computer & Electrical Engineering), a notable expert in PAI, we selected a number of chromophores that possessed NIR absorption characteristics. This allowed a unique side-by-side comparison of these dyes as PAI contrast agents. Using a quinoline-annulated porphyrin vs. the benchmark dye indocyanine green (ICG) or blood shows a 2.5-fold contrast enhancement of a very small tube (translucent polyethylene tube, Dinner = 0.38 mm) filled with the chromophore solutions in a tissue phantom at 1 and 2 cm depths.
Light Harvesting
Technical applications will also benefit from the long-wavelength absorbance characteristics of novel porphyrinoids. For instance, artificial photosynthetic systems may improve their efficiency through the ability to absorb red and NIR light which constitutes an appreciable portion of sunlight. In collaboration with the group of Prof. Alex Agrios (UConn Department of Civil and Environmental Engineering), we are evaluation the use of our porphyrins and porphyrinoids as light-harvesting pigments in dye-sensitized solar cells, so-called Grätzel cells. We are working on generalized strategies to attach the dyes to the semiconductor surface.
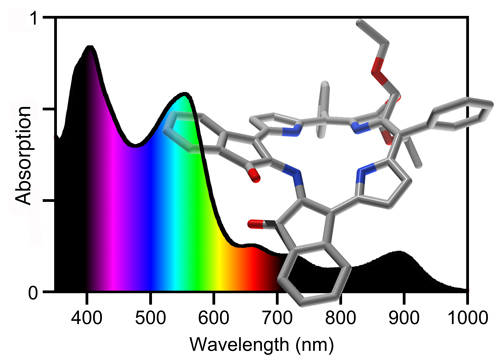
Consider, for example, the spectra of the indachlorins, PMPs with particularly panchromatic optical spectra (their suitability as light harvesting dyes is as yet not known).
PMP Cobalt(II) Complexes as Catalysts for the Electrochemical Reduction of CO2
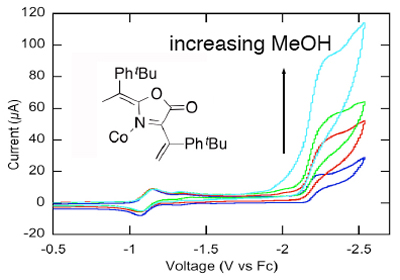
The capture of CO2 and its conversion to useful chemicals is a timely field of research. The photo-chemical or electrochemical reduction of CO2 to, for example, HCO2H, CO, or even CH3OH is particularly interesting as renewable energy resources can be used to drive these reactions. However, the direct reduction of CO2 is highly unfavorable, requiring high potentials. (Proton-assisted) multi-electron transfer reactions are more favorable but require efficient catalyst, such as cobalt meso-tetraarylporphyrin complexes, which possess modest activity and turn-over-number.
In collaboration we Prof. Alfredo Angeles-Boza (UConn Chemistry), we prepare and screen Co(II) PMP complexes to help delineate the structural requirements (redox state of the ring, conformation, and conformational flexibility) for efficient (low potential and fast rate constants) Co(II) PMP-catalyzed CO2 reduction. The influence of intramolecular proton-assisted catalysis will be tested also.
Chemosensing
Our work using porphyrinoids and other molecules in chemosensing applications is described here.