The General Aim of the Brückner Porphyrinoid Research
 We aim at synthesizing porphyrinoid molecules that possess much altered electronic properties than what is found in naturally occurring systems. Our principle tool – the Brueckner Way, if you will – is the conversion of a pyrrolic moiety of a porphyrin through step-wise modifications into a non-pyrrolic moiety. The guiding hypothesis of the work is that these modifications result in drastically altered electronic properties compared to porphyrins or chlorins. This data will be correlated with their structural parameters. The codification of the structure-relationships of pyrrole-modified porphyrins will help to design porphyrinic chromophores with designed photophysical properties. Long wavelength (near-IR range) absorbing and fluorescing chromophores promise to be of utility in the biomedical field as one- and two-photon imaging or photo-therapeutic (PDT) agents. We aim at increasing their fluorescence yields, photoacoustic response profiles, or carrying functionalities at their periphery that allow their utilization in sensing applications. Our work is further detailed here.
We aim at synthesizing porphyrinoid molecules that possess much altered electronic properties than what is found in naturally occurring systems. Our principle tool – the Brueckner Way, if you will – is the conversion of a pyrrolic moiety of a porphyrin through step-wise modifications into a non-pyrrolic moiety. The guiding hypothesis of the work is that these modifications result in drastically altered electronic properties compared to porphyrins or chlorins. This data will be correlated with their structural parameters. The codification of the structure-relationships of pyrrole-modified porphyrins will help to design porphyrinic chromophores with designed photophysical properties. Long wavelength (near-IR range) absorbing and fluorescing chromophores promise to be of utility in the biomedical field as one- and two-photon imaging or photo-therapeutic (PDT) agents. We aim at increasing their fluorescence yields, photoacoustic response profiles, or carrying functionalities at their periphery that allow their utilization in sensing applications. Our work is further detailed here.
Porphyrins
 Porphyrins are cyclic, tetrapyrrolic, and strongly colored compounds. Their name is derived from the Greek word for purple (porphyros). Naturally occurring, porphyrins are the parent members of the Pigments of Life, play key roles in metabolic processes and photosynthesis. Heme, for instance, the red pigment in blood, is an iron complex of a porphyrin (protoporphyrin IX). Hemes are also the crucial factors in enzymes as diverse as oxidases (such as some liver enzymes metabolizing drugs), catalases (that protect the cell from oxidative damage), or electron transfer proteins.
Porphyrins are cyclic, tetrapyrrolic, and strongly colored compounds. Their name is derived from the Greek word for purple (porphyros). Naturally occurring, porphyrins are the parent members of the Pigments of Life, play key roles in metabolic processes and photosynthesis. Heme, for instance, the red pigment in blood, is an iron complex of a porphyrin (protoporphyrin IX). Hemes are also the crucial factors in enzymes as diverse as oxidases (such as some liver enzymes metabolizing drugs), catalases (that protect the cell from oxidative damage), or electron transfer proteins.
Hydroporphyrins (Chlorins)

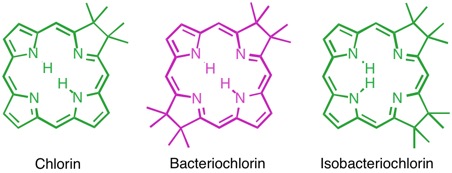
 Hydroporphyrins are reduced porphyrins. Particularly important are the class of chlorins. Chlorins, in the form of Mg2+ complexes, are found as the green photosynthetic pigments in plants (chlorophylls). The Mg2+ complexes of tetrahydroporphyrins of the bacteriochlorin class are, for instance, the light-harvesting chromophores of, for instance, phototrophic purple bacteria, heliobacteria, or green sulfur bacteria (bacteriochlorophylls). The iron complex of the isomeric isobacteriochlorin, siroheme, is the prosthetic group in sulfite and nitrite reductases.
Hydroporphyrins are reduced porphyrins. Particularly important are the class of chlorins. Chlorins, in the form of Mg2+ complexes, are found as the green photosynthetic pigments in plants (chlorophylls). The Mg2+ complexes of tetrahydroporphyrins of the bacteriochlorin class are, for instance, the light-harvesting chromophores of, for instance, phototrophic purple bacteria, heliobacteria, or green sulfur bacteria (bacteriochlorophylls). The iron complex of the isomeric isobacteriochlorin, siroheme, is the prosthetic group in sulfite and nitrite reductases.
The importance of chlorins has spawned a wide range of methodologies to generate synthetic chlorins and bacteriochlorins, many of which are oxidatively much more stable than their natural counterparts. The methods range from the conversion of synthetic or naturally occurring porphyrins and chlorins to chlorins and bacteriochlorins, to the total synthesis of these chromophores. For an overview on the state of the art in the synthesis of bacteriochlorins, see Brückner, C.; Samankumara, L.; Ogikubo, J. ‘Syntheses of Bacteriochlorins and Isobacteriochlorins’ in Handbook of Porphyrin Science; Kadish K. M.; Smith, K. M.; Guilard, R., Eds.; World Scientific: River Edge, NY, 2012; Vol. 17, pp. 1–112.
Optical Spectra of Porphyrins and Chlorins
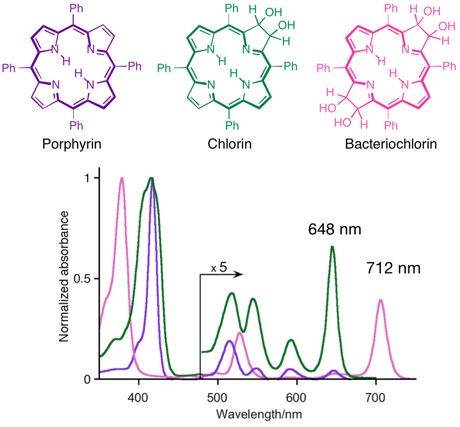
The consecutive reduction of one or two ß,ß’-double bonds of a porphyrin has dramatic consequences on their optical spectra. They each adopt very characteristic and increasingly long wavelength shifted spectra, as demonstrated by the comparison of the three corresponding mess-tetraaryl-substituted chromophores:
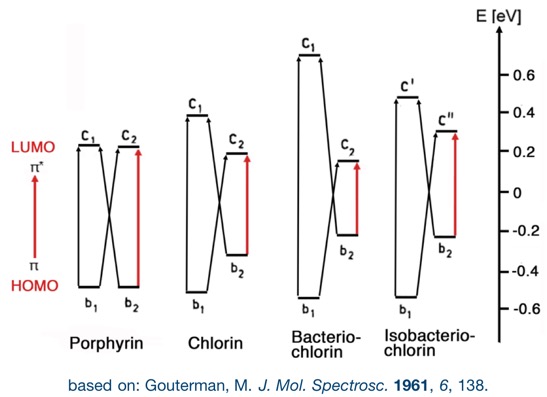
This change in their electronic properties is rationalized by their change of the energy and symmetry of their frontier orbitals, as explained by the Gouterman four orbital model. In particular, the red-shift is rationalized by a narrowing HOMO-LUMO gap with increasing reductions. Any other reaction that removes the ß,ß’-double bond from conjugation elicit these changes to the optical spectra. Substituents and the modulation of the – by default planar – conformation and conformational flexibility of the chromophores further influence the photo physical properties of the chromophore.
Applications of Porphyrins and Chlorins
Due to their strong interaction with light, their relative high chemical stability, porphyrins and chlorins found an enormously wide range of applications, ranging from their use as oxidation, other atom transfer, and electron transfer catalysts, in environmental remediation projects, as synthetic light harvesting dyes, in information storage technologies, as photochemotherapeutics in cancer treatment, as photoantimicrobials, as fluorescent and photoacoustic imaging dyes in biomedical fields, as chemosensors, and as building blocks. Moreover, their fundamental study continues to harness insight into the working of crucial enzymes in our body or the photosynthetic apparatus. For examples of the applications we target, see here.
However, most of these applications require a much broader tuning of their photophysical properties (colors of the absorbed or emitted light, etc.) than can be accomplished by a mere reduction.